Medical
Increase everyone's accountability
The responsabilities of a medical controller within the EFPIA legislation are huge. TransPharmacy supports the Medical department by guiding sales people towards the proper documents and contracts and by creating complete transparancy over the entire promotional process.
Every step in the proces is traceable: who, when, what, where filled out a piece of information, gave his/her approval, signed a document, requested a budget, ...
TransPharmacy is strict with legal requirements. Each type of promotional activiy has a predefined flow in which the appropriate actor provides information or gives an approval.
Only when all necessary information is present (e.g. MDEON, RIZIV/INAMI, presentations, ...), will the system present the request to the medical controller for approval. No more semi-completed documents, missing beneficiaries, illegible data.
Request a demo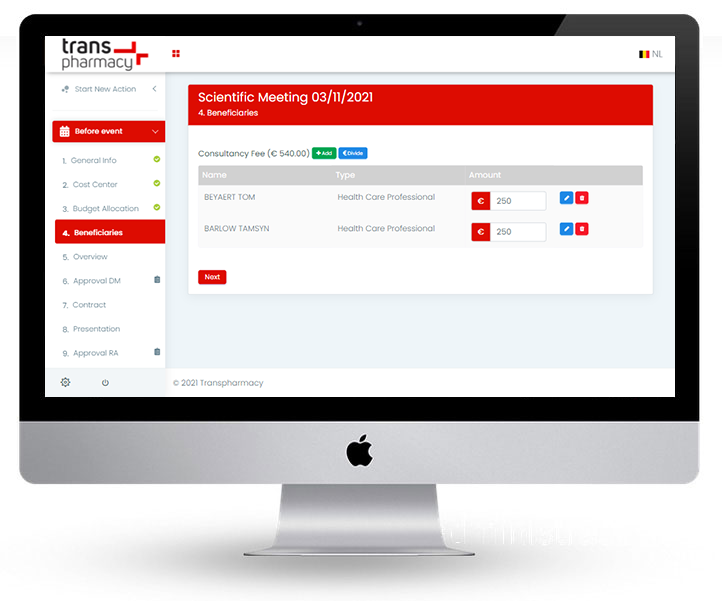
- One central storage of all legal documents and requirements.
- Through transparancy, everybody in the process is accountable.
- Re-use and connect with official databases for HCP-data, product offer, ...
- Clear overview on the dashboard of actions that require your approval.
- Strong control on legal requirements.